What we do
Since May of 2014 the Laboratory of EPR spectroscopy does not exist anymore in the form presented on these pages.Members of the former staff are still involved in EPR spectroscopy research though.
The spectrometer and its accessories available at the laboratory enable us to perform a high variety of X-band EPR spectroscopy measurements and to address diverse scientific problems in the fields of physics, chemistry and biology. By the means of EPR spectroscopy, in these fields one can often find answers to relevant scientific questions via the deliberate application of routine measurement procedures. At the same time, on numerous occasions it requires a great deal of creativity to establish a proper way of proceedings that will lead to the desired results. To describe all the different ways EPR spectroscopy can contribute to the solution of scientific problems is far out of our scope here, but in order to introduce some of the ways we typically handle scientific problems via EPR spectroscopy, we nevertheless describe here a few of our achievements and case studies from the recent (as well as not-so-recent) past.
Our computer softwares for the handling, simulation and fitting of EPR spectrum data
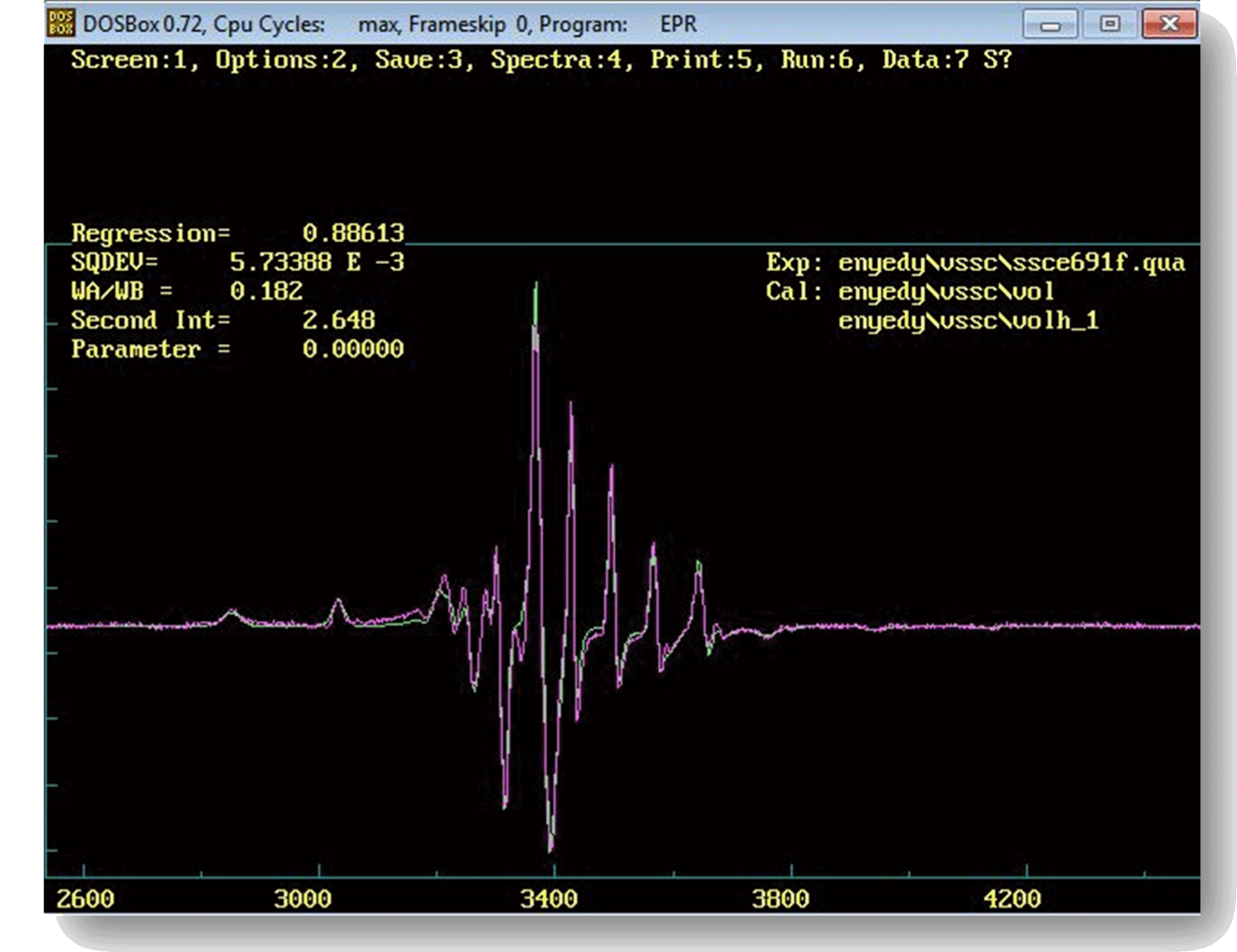
EPR spectra in practice can be quite complex, consequently their quantitative analysis and interpretation usually requires a rather serious effort. As the laboratory has considerable experience in computer programming as well as in EPR theories, whenever it is reasonable we prefer to develop and use our own software tools for data handling and analysis. The computer programs we most often utilize to analyze all kinds of EPR spectra of, e.g., free radicals and metal complexes have their origin in developments started back in the 1990's (1996) and continued ever since with new theories and options added on a regular basis as required by the scientific problems under study (see, e.g., 2001). Though originally developed for the DOS operating system, these programs run readily in DOSBox even under modern MS Windows and Linux OSs (see image on the right), and are invaluable tools in practice on account of their wide range of unique capabilities that make them able to calculate theoretical—and to fit corresponding measured—EPR spectra for a wide range of experimental situations. They can for example readily handle liquid as well as frozen/solid samples with components displaying either isotropic or anisotropic interactions, can account (and can provide the corresponding correlation time) for dynamic effects ranging from the slow to the fast motional regime, and are able to model the effects of pH, temperature, concentrations and rate constants in the case of—so called two-dimensional—spectrum series recorded of solutions (see, e.g., 2006). A further development of these program codes is also in progress with the aim to describe EPR spectra of systems with coupled spins (e.g., biradicals and dinuclear transition metal complexes).
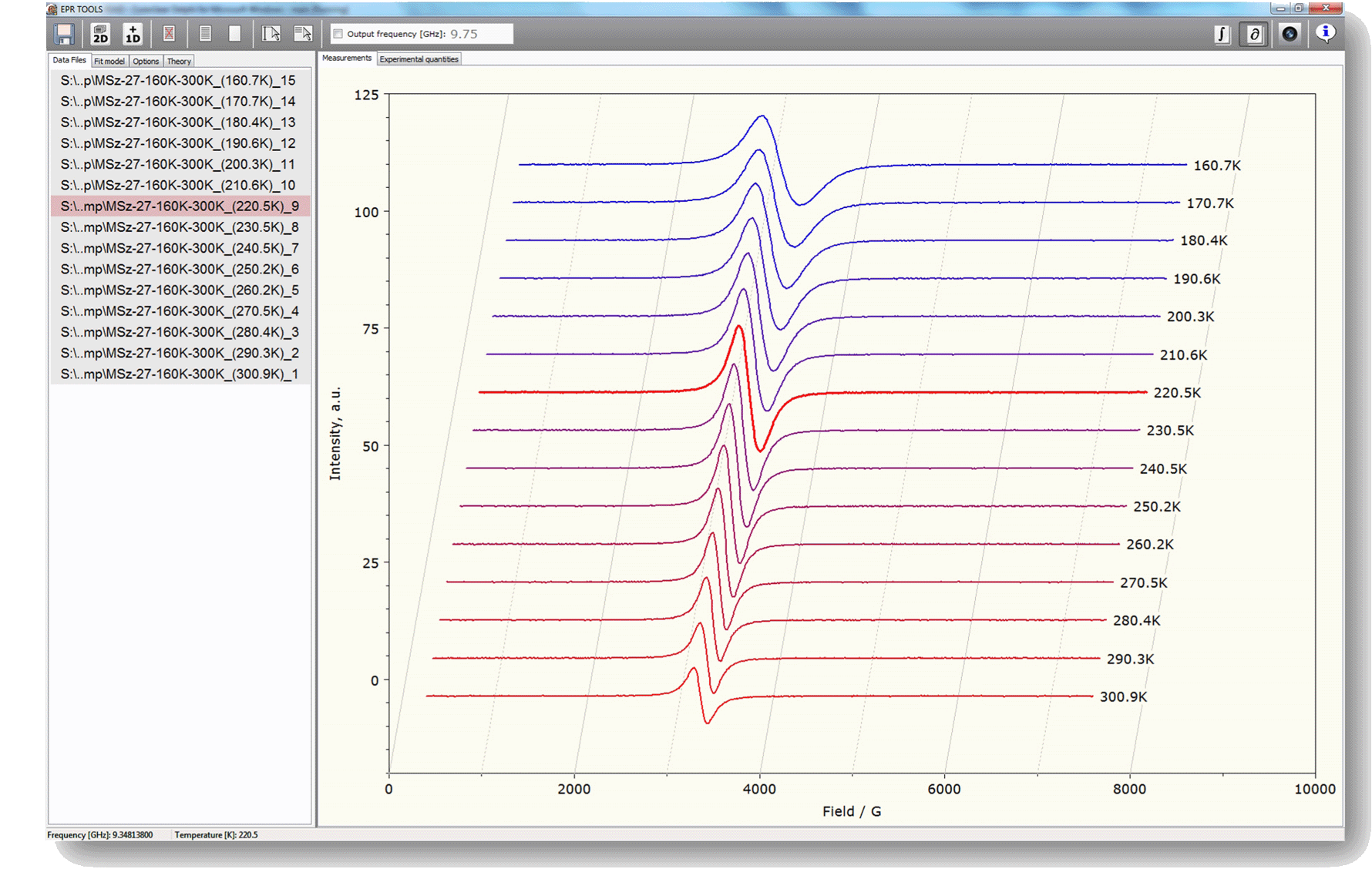
Initiated by the extraordinary computational power required by the utilization of some theories modeling molecular reorientational processes in liquids, we have also recently set out to develop native program codes for the MS Windows operating system, that can utilize the computational power of modern multicore processors, e.g., by applying parallel computing techniques in order to achieve a significant increase in the computing speed. By developing native code for MS Windows it is also our aim to take advantage of the advanced graphical and internet functions offered by modern operating systems. An early version of the corresponding computer software is already in use especially for some everyday data handling and graphing procedures (see image on the right).
In addition, we have several further program codes that enable us to analyze FMR (ferromagnetic resonance) spectra of powders of ferromagnetic particles displaying either uniaxial or cubic magnetocrystalline anisotropy.
References
- A. Rockenbauer, N.V. Nagy, F. Le Moigne, D. Gigmes, P. Tordo:
Thermodynamic Analysis of the Chemical Exchange of β-Phosphorylated Cyclic Nitroxides by Using Two-dimensional (Temperature versus Magnetic Field) Simulation of ESR Spectra: The Impact of Labile Solvent-Solute Interactions on Molecular Dynamics,
J. Phys. Chem. A 110 (2006) 9542-9548. - A. Rockenbauer, T. Szabó-Plánka, Zs. Árkosi, L. Korecz:
A two-dimensional (magnetic field and concentration) electron paramagnetic resonance method for analysis of multi-species complex equilibrium systems. Information content of EPR spectra,
J. Amer. Chem. Soc. 123 (2001) 7646-7654. - A. Rockenbauer, L. Korecz:
Automatic Computer Simulations of ESR Spectra,
Appl. Magn. Reson. 10 (1996) 29-43. - A. Rockenbauer, P. Simon:
Perturbation solution of the spin Hamiltonian of low symmetry using the projector technique,
Mol. Phys. 28 (1974) 1113.
Paramagnetic metal complexes in aqueous solutions
|
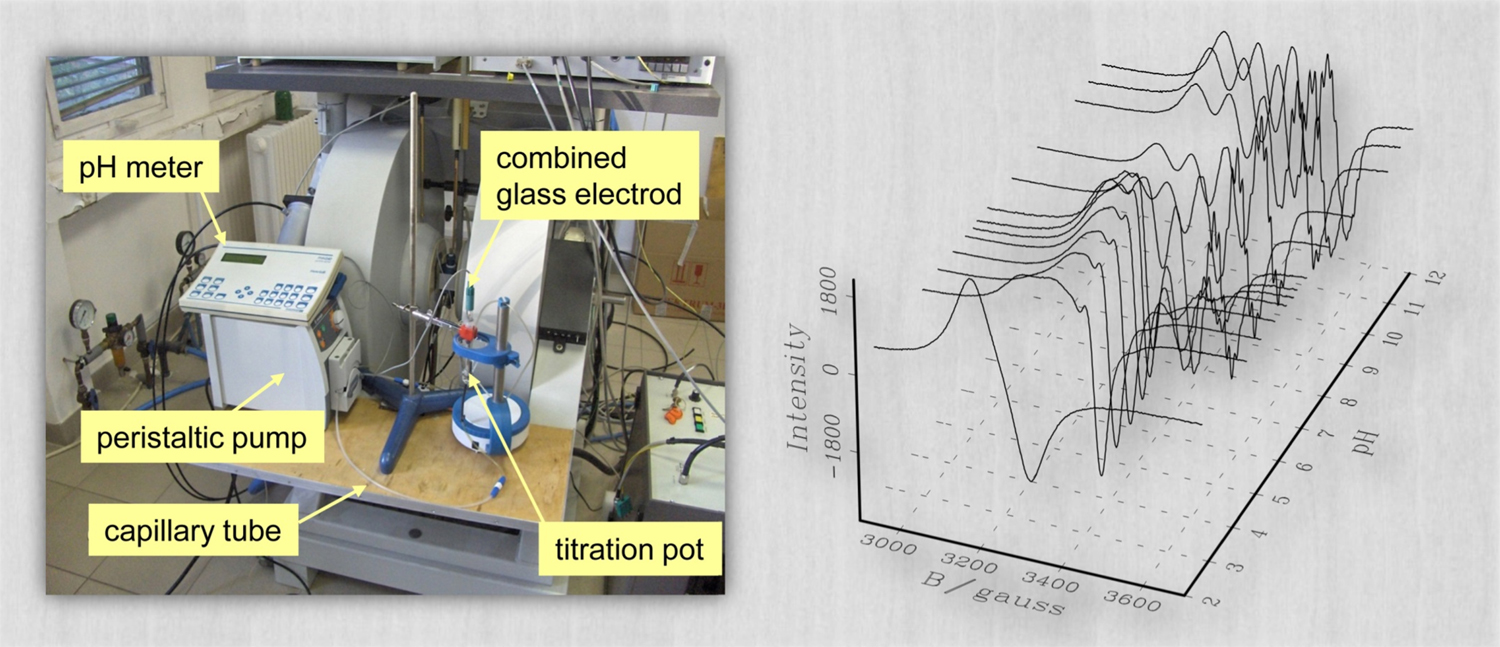
Investigations of solution equilibrium systems of metal complexes with biologically relevant (bio)molecules are prompted by different research areas such as (1) the study of structure-effect relations of anticancer metal drugs, (2) the development of chelate therapy in metal accumulation related (e.g., Alzheimer) diseases, and (3) the study of the chelation of metal ions for the purposes of medical imaging (contrast agents) and therapy (radionuclides). For complexes with potential biological relevance, it is of crucial importance to assess the molecular and electronic structure and the stability thereof under experimental situations modeling physiological conditions. In case of paramagnetic metal complexes, EPR spectroscopy is one of the most powerful method to accomplish this task. In aqueous solutions of metal ions and ligands several complexes with different compositions or coordination modes may form at different pHs and concentrations, depending on the protonation state of the ligands. As the interconversion rate of these species is rather slow on the EPR timescale ( ∼1 ns for X-band ), the measured spectra are the sums of the individual spectra of the different species, weighted by their concentration in the solution. In such solution equilibrium systems the accurate quantitative decomposition of EPR spectra enables one to determine the formation constants characterizing the different individual species. To reach a suitable level of accuracy, EPR spectra are recorded under the same instrumental settings and geometrical conditions, which is achieved by using a circulating system to perform in situ titration with a strong base solution. In this setup (see the image above), a peristaltic pump ensures the circulation of the titrated solution through the capillary tube, which latter is led across the measuring cavity. In the two-dimensional approach to spectrum analysis, the measured spectra (representing the EPR signal intensity as a function of the sweeping magnetic field) are also treated as a function of the concentration of the individual species, and the series of pH-dependent EPR spectra recorded at different metal-to-ligand concentration ratios are analyzed simultaneously by fitting the formation constants (defined in the mass-balance equations) along with the EPR parameters for all the paramagnetic species. (See also a corresponding poster in PDF.)
References
- A. Lakatos, B. Gyurcsik, N.V. Nagy, Z. Kele, Z. Csendes, Cs. Triznya, L. Fülöp, T. Kiss:
Histidine-rich branched peptides as Zn(II) and Cu(II) chelators with potential therapeutic application in Alzheimer’s disease,
Dalton Trans. 41 (2012) 1713-1726. - N.V. Nagy, S. Van Doorslaer, T. Szabó-Plánka, S. Van Rompaey, A. Hamza, F. Fülöp, G.K. Tóth, A. Rockenbauer:
Copper(II)-binding ability of stereoisomeric cis- and trans-2-aminocyclohexanecarboxylic acid – L-phenylalanine dipeptides. A combined CW/pulsed EPR and DFT study,
Inorganic Chemistry 51 (2012) 1386-1399. - É.A. Enyedy, N.V. Nagy, É. Zsigó, C.R. Kowol, V.B. Arion, T. Kiss, B.K. Keppler:
Comparative solution equilibrium study on interaction of copper(II), iron(II) and zinc(II) with Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone) and related ligands,
Eur. J. Inorg. Chem. 11 (2010) 1717-1728. - N.V. Nagy, T. Szabó-Plánka, A. Rockenbauer, G. Peintler, I. Nagypál, L. Korecz:
Great Structural Variety of Complexes in Copper(II) - Oligoglycine Systems. Microspeciation and Coordination Modes as Studied by the Two-Dimensional Simulation of Electron Paramagnetic Resonance Spectra,
J. Am. Chem. Soc. 125 (2003) 5227-5235.
Aqueous solutions of macromolecules and oligopeptides
|
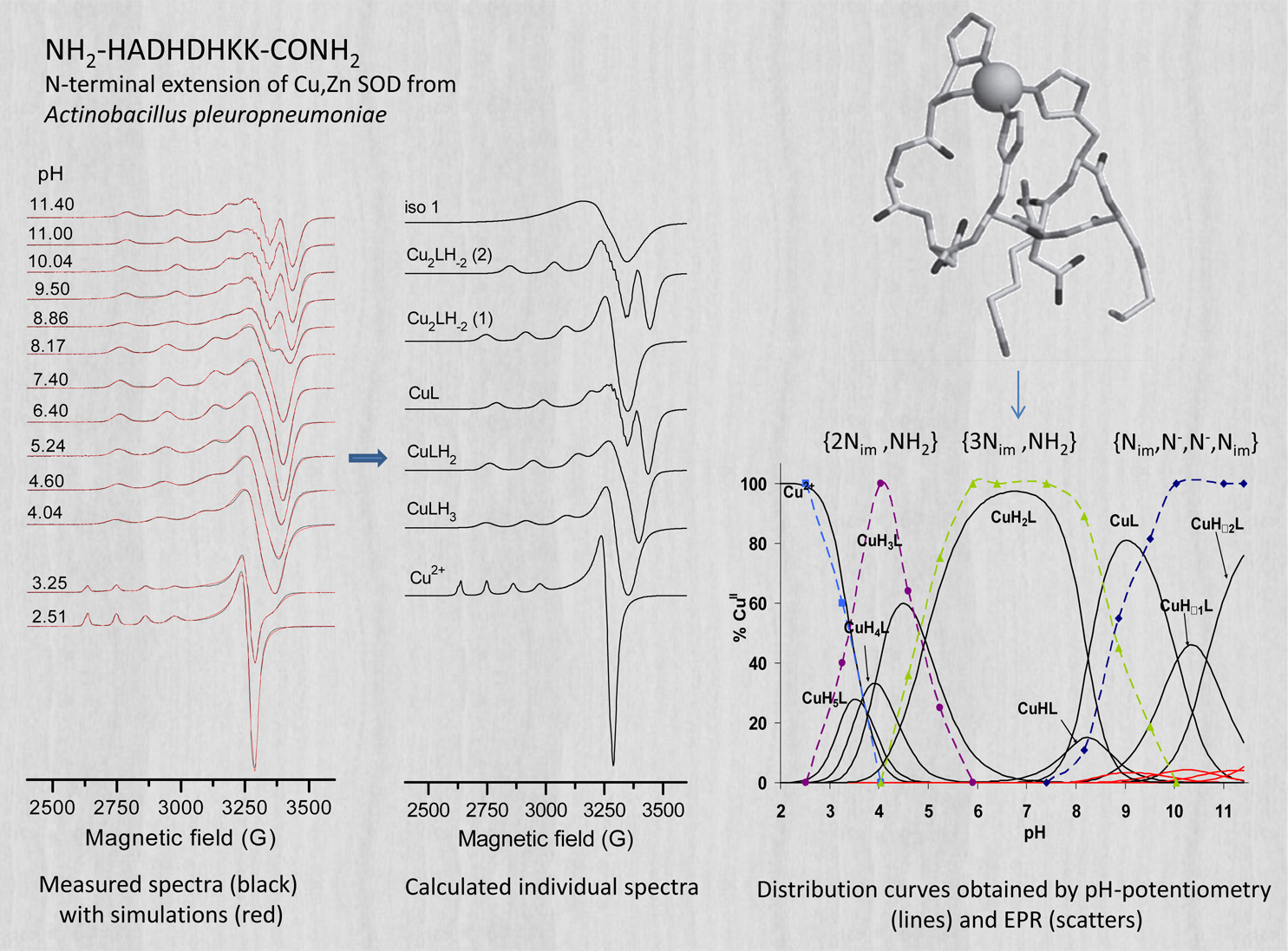
Owing to their relatively high molecular mass, macromolecules and oligopeptides can display a rather slow molecular motion in their room-temperature solutions, which leads to EPR spectra with a peculiar spectral shape that cannot be described satisfactorily with the usual averages characterizing either the low-temperature (static) or the high-temperature (motionally narrowed, fast-motion) limits. To observe detailed information about the geometry, coordination properties and concentration of such molecular species, it is therefore often advantageous to investigate rather their frozen solutions whose EPR spectra can be readily recorded and decomposed into the individual (static limit) spectra of the constituent species (see image on the right). In addition, the combined application of (low-temperature) EPR spectroscopy and (room-temperature) pH potentiometry can help to identify situations where the composition of a corresponding solution and/or the structure of the molecules differ in the liquid and frozen solution phases, and may reveal the presence of different species with very similar coordination modes, which latter usually all contribute to the same EPR signal. (See also a corresponding poster in PDF.)
References
- D. Árus, Á. Dancs, N.V. Nagy, T. Gajda:
A comparative study on the possible zinc binding sites of the human ZnT3 zinc transporter protein
Dalton Transaction 42 (2013) 12031-12040. - D. Árus, N.V. Nagy, Á. Dancs, A. Jancsó, R. Berkecz, T. Gajda:
A minimalist chemical model of matrix metalloproteinases — Can small peptides mimic the more rigid metal binding sites of proteins?
J. Inorg. Biochem. 126 (2013) 61-69. - D. Árus, A. Jancsó, D. Szunyogh, F. Matyuska, N.V. Nagy, E. Hoffmann, T. Körtvélyesi, T. Gajda:
On the possible roles of N-terminal His-rich domains of Cu,ZnSODs of some Gram-negative bacteria
J. Inorg. Biochem. 106 (2012) 10-18. - A. Jancsó, A. Kolozsi, B. Gyurcsik, N.V. Nagy, T. Gajda:
Probing the Cu2+ and Zn2+ binding affinity of histidine-rich glycoprotein,
J. Inorg. Biochem. 103 (2009) 1634-1643.
Study of molecular dynamics
|
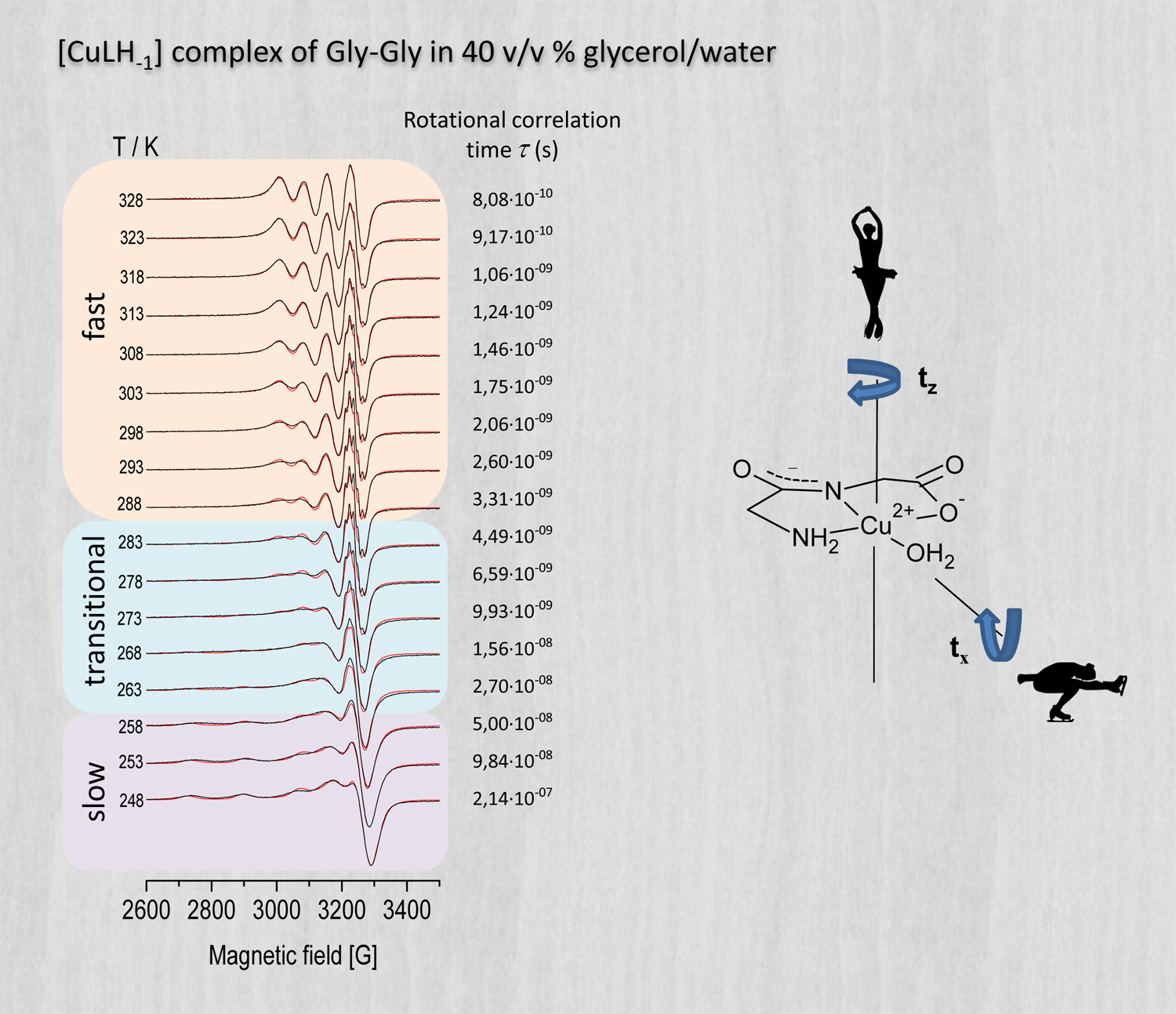
X-band EPR spectroscopy is a great tool to study molecular dynamics and associated phase transitions. The sensitivity of X-band EPR to molecular dynamics is due to the advantageous intrinsic measurement time window of the method, which is in the order of 1 ns ( 10–9 s ). Dynamic molecular processes with a period considerably shorter than this time will result in an EPR spectrum where only the motional average of relevant interactions (represented by the g-tensor and the hyperfine coupling tensor) become visualized (motional narrowing, leading to so called isotropic spectra). This is the usual case for ordinary solutions at room temperature, where the rotational/reorientational correlation time of molecules lies typically in the order of a few picoseconds ( 10–12 s ), and thus during the EPR transition the molecules turn round about a few hundred times. When the period of rotation/reorientation of molecules considerably exceeds the intrinsic time window (i.e. when the period is above ∼100 ns), a random powder like, static-limit EPR spectrum is observed, as if the molecules were stationary during the EPR transition. In the intermediate period range the spectral shape of X-band EPR spectra is especially sensitive to changes in the rotational/reorientational correlation time of molecules, and can therefore reveal corresponding phase transitions in a direct and clear way few other experimental methods can match. The analysis of the transitional EPR spectral shapes obtained in this most sensitive period range can yield the corresponding rotational/reorientational correlation time of the molecules under study. Whereas spin labels such as nitroxide radicals (introduced into biological systems) are widely used to investigate dynamical properties of, e.g., proteins, paramagnetic transition metal complexes can also be used for the study of molecular dynamics in a similar manner. In the example above (see image) our analysis for example revealed that the molecule rotates faster around its z axis than around its x and y axes.
Detecting phase transitions in phospholipid bilayers
|
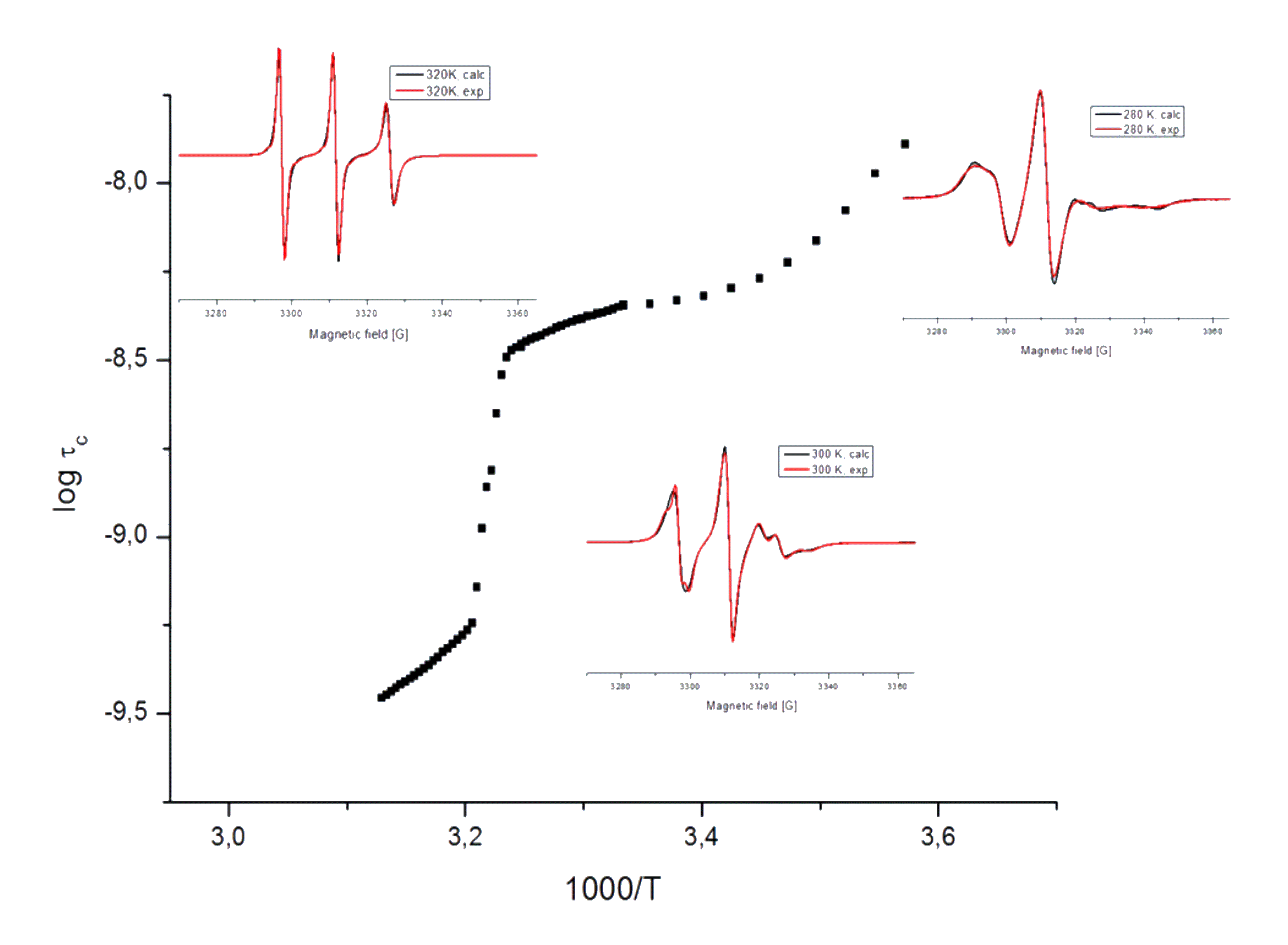
The study of lyotropic liquid crystal features of biomembrane-forming phospholipid bilayers is an accentuated topic of soft condensed matter physics. Liposomes (supramolecular structures formed by the self-assembly of amphiphilic lipid molecules) are widely used in fundamental biomembrane research as well as in biotechnological development. During the production of model membranes the type and concentration of lipids are readily variable, and it is possible to study how various guest molecules influence the structure and stability of the membrane. As the lipid molecules do not provide an EPR signal in themselves, usually spin labels are used to study their molecular dynamical properties. As spin label, in our study we have used a nitroxyl free radical attached to the 16th carbon atom of a bilayer-forming stearic acid through a doxyl group (16-DSA), and prepared a solution of a mixture of labeled and unlabeled (DPPC) lipid molecules with a molar ratio of 1:100, with the aim to monitor the dynamic properties of the inner (hydrophobic) zone of the formed membranes. On the basis of a series of X-band CW-EPR spectra—recorded in the temperature range of 280-320 K—we have established the temperature dependence of the rotational correlation time ( τc ) of the labeled molecules. The results (see image above) clearly reveal the occurrence of a pronounced phase transition of the membranes at ∼310 K, as well as a milder one at ∼285 K. The phase transition at ∼310 K is connected to a rearrangement of the lipid layers, which leads to a sudden order-of-magnitude drop in the rotational correlation time, and consequently to a more free rotation/reorientation of the spin labels at higher temperatures. (See also a corresponding poster in PDF.)